Her pulmonologist, following established clinical guidelines for advanced respiratory management, transitioned her to a newer dual bronchodilator therapy combining indacaterol and glycopyrronium. Delivered via inhaled capsule, the medication was designed to improve lung function, reduce exacerbations, and enhance quality of life. It was considered low-risk, localized, and far safer than systemic alternatives. Two days after starting the new inhaler, the first symptoms appeared.
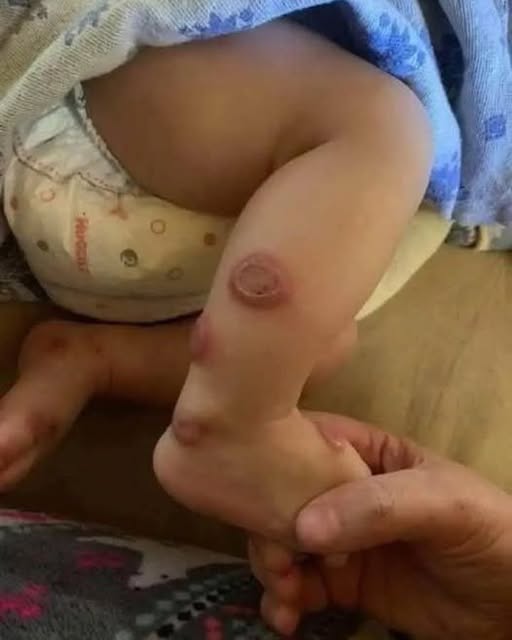
She developed sharply defined, erythematous plaques across her face and neck. The lesions were painful, warm to the touch, and unlike anything she had experienced before. Alongside the skin changes came a low-grade fever and a deep sense of malaise that could not be dismissed as fatigue. She reported no new skincare products, no dietary changes, no recent infections, and minimal sun exposure. She had used sunscreen consistently. Nothing in her routine explained the sudden inflammatory response.
Recognizing that the rapid onset and severity of the lesions suggested something more than a cosmetic issue, her primary care physician acted decisively and referred her to dermatology for urgent evaluation.
That decision would prove critical.
At the dermatology clinic, the team immediately suspected a systemic inflammatory process rather than a superficial skin condition. The presentation was aggressive, painful, and accompanied by constitutional symptoms. They recommended immediate discontinuation of the new inhaler, initiation of systemic corticosteroids, comprehensive laboratory testing, and a skin biopsy from an active lesion. Within forty-eight hours of stopping the medication and starting treatment, the patient began to improve. The redness faded. The pain subsided. Her fever resolved. The skin, once inflamed and angry, began to calm.
The laboratory results told a clearer story. Bloodwork revealed marked leukocytosis with significant neutrophilia, a red flag for acute inflammatory syndromes. An extensive autoimmune panel was ordered to rule out connective tissue diseases and systemic lupus erythematosus. Antinuclear antibodies, anti-dsDNA, lupus anticoagulant, and other markers all came back negative. There was no evidence of vasculitis. No malignancy was detected. No infection explained the immune response.
Twenty days later, the skin biopsy confirmed the diagnosis: Sweet’s syndrome, formally known as acute febrile neutrophilic dermatosis.
Sweet’s syndrome is a rare and often misunderstood inflammatory condition characterized by the sudden appearance of painful, erythematous plaques or nodules, accompanied by fever, elevated white blood cell counts, and a distinctive histopathological finding of dense neutrophilic infiltration in the dermis without vasculitis. It is most commonly associated with infections, autoimmune disorders, hematologic malignancies, or medications—typically systemic drugs such as antibiotics, antiepileptics, or colony-stimulating factors.
What made this case extraordinary was the trigger.
There were no prior documented cases clearly linking indacaterol/glycopyrronium inhaled therapy to Sweet’s syndrome. Inhaled medications are generally considered to have minimal systemic immunologic impact due to their localized delivery. Yet in this patient, the immune system responded as if it had been profoundly provoked. The presumed trigger was not an oral or injectable drug, but a bronchodilator capsule inhaled directly into the lungs.
The differential diagnosis had initially included allergic contact dermatitis, phototoxic reactions, drug-induced toxicoderma, lupus erythematosus, and even severe rosacea with systemic involvement. However, the combination of clinical presentation, laboratory findings, rapid steroid response, and biopsy results allowed clinicians to rule these out with confidence.
Treatment followed established protocols for Sweet’s syndrome: removal of the offending agent and a short course of oral corticosteroids. The response was swift and complete. Within one week, the lesions resolved entirely. No scarring remained. Follow-up visits showed no recurrence, and alternative COPD management was implemented without further complications.
Beyond the individual outcome, the case carries significant implications for clinical medicine, particularly in primary care, pulmonology, and dermatology. It challenges long-held assumptions about the systemic safety of inhaled therapies and underscores the importance of vigilance when evaluating new-onset skin symptoms following any medication change, regardless of delivery method.
This case reinforces several critical lessons. Sweet’s syndrome must remain in the differential diagnosis when patients present with sudden, painful inflammatory skin lesions accompanied by systemic symptoms. A thorough medication history—including recent changes, formulation switches, and delivery mechanisms—is essential. Early dermatological referral and prompt biopsy can be lifesaving, preventing unnecessary progression and guiding effective treatment. Most importantly, clinicians must remember that rare immune-mediated reactions can arise from unexpected sources.
The ethical considerations were carefully addressed. No experimental procedures were performed. Patient confidentiality was fully protected. Written informed consent was obtained for publication of the case, adhering to established clinical and ethical standards .
In the broader context of modern medicine, this case stands as a reminder that the body communicates in subtle ways before it fails loudly. The skin, often dismissed as superficial, can be the first organ to signal deep systemic distress. For healthcare professionals navigating an era of advanced pharmacology and complex therapies, listening to those signals remains as vital as ever.
Sometimes, a red rash is not just a rash. Sometimes, it is the immune system sounding an alarm. And in this instance, that alarm came not from the lungs—but from the skin.